Why New Analgesics Fail: Patient Stratification
Pain R&D forms the foundations for the entire pain management process, with these initial decisions key to whether pain treatments succeed or fail.
The truth is, the system is flawed and, ultimately, the majority of new analgesics fail at the clinical development stage.
But why is this?
While there are a number of factors at play, one key aspect lies in an outdated approach and a lack of true innovation that focuses on a limited number of pain patient groups. This contributes to an over-reliance on pre-clinical models and fixed regulatory pathways that fail to account for the complex and varied nature that is chronic pain.
Chronic pain with moderate to severe intensity affects around 20% of the US population¹. This equates to millions of people suffering due to the limitations associated with traditional pain R&D methods.
Researchers tell us² that the knowledge and tools are available to increase the efficacy of pain treatment but, despite this, the industry continues to cling to conventional R&D methods, leaving countless patients underserved.
So what’s the solution?
In this article, we detail how this lack of innovation and precision causes problems for pharmaceutical companies and patients alike and explore how the Pain Cloud® platform can help solve these challenges.
The problems with limited pain research
Despite the fact there is a huge resource of molecular biology databases available, traditional R&D practices often remain focused on just a select number of patient groups representing the biggest market value, failing to consider the impact huge number of patients suffering from other pain conditions.
This reluctance to include diverse patient groups results in billions of people being disregarded during the R&D stage of developing new analgesics.
This is a significant contributing factor in the shockingly low clinical success rate of new drugs – which stands at under 1%.
Even within the limited number of patient groups being used by the industry there are issues resulting in a lack of precision analgesics. This is because these groups are highly complex and heterogenous conditions but are often treated as homogenous within clinical development diluting possible treatment effects.
Take this example…
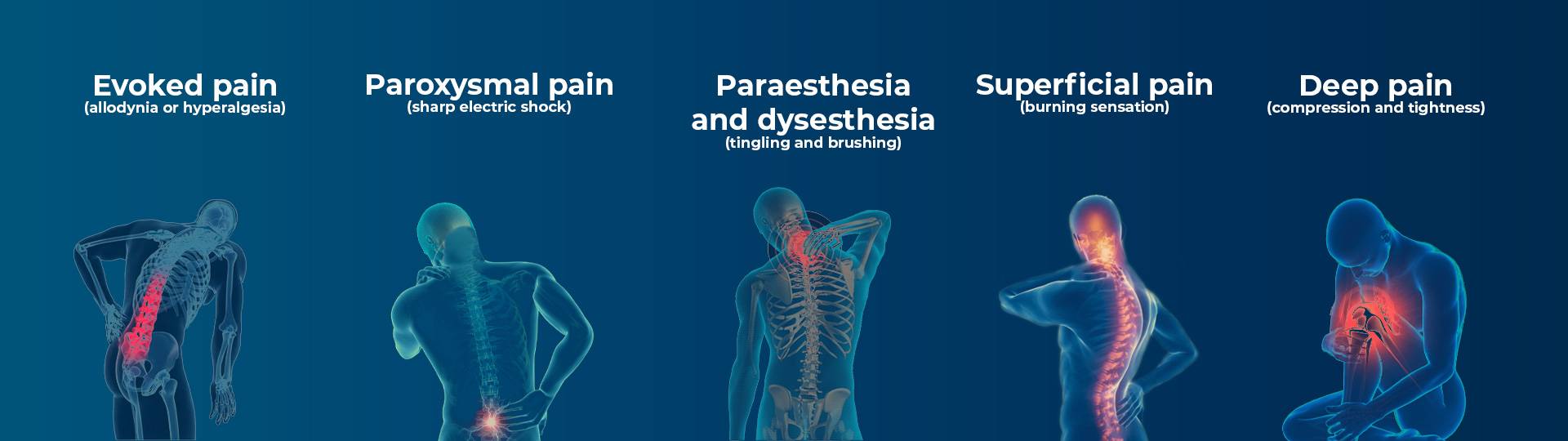
Diabetic neuropathy is the main patient population utilized for neuropathic pain and patients are rarely stratified with clinical protocols. However, there are multiple types of peripheral diabetic neuropathy, each with the potential to present themselves very differently in individuals with diabetes.
As of 2021, there were around 415 million adults with diabetes in the world, 16%-26% of which present neuropathic pain which is consistent or intermittent for more than three months. But current medicines do not take into account the different subpopulations of diabetic neuropathy, meaning many people with the condition are not receiving optimal treatment.
Clinical pain symptoms are all different and, while each individual cannot be treated independently based on their individual experiences, R&D should be focusing on these pain phenotypes:
Making pain research more inclusive
One of the key reasons traditional pain R&D lacks precision is the fact that it often excludes large groups of people.
Inclusive pain research practices, i.e. ones that focus on people of a variety of ages, racialized groups or ethnicities, disabilities, gender identities and sexual orientations, are imperative to ensuring everyone is treated as fairly as possible. This enables subsequent treatments and advice to meet the needs of society as a whole, not just within select groups.
Identifying different subgroups
During trials, systematic reviews and meta-analyses, broad conditions are often regarded as homogenous categories, meaning that true treatment effects may be diluted when only a subgroup of patients respond.
To achieve the best possible treatment, research must focus on specific pain phenotypes.
But how can this be achieved?
- Quantitative sensory testing
This could involve, for example, neuropathic pain patients and fibromyalgia patients being classified into broad phenotypes.
Quantitative sensory testing (QST) enables us to measure changes in sensitivity to different types of sensations such as pressure, touch or temperature. This helps categorize patients more accurately.
- Targeted interview questions
One of the simplest ways to gather accurate data with which to make decisions is by asking patients directly.
For example, this survey³ studies targeted interview responses from different subgroups suffering chronic pain from spinal cord injuries.
The report shows that different subgroups responded differently to certain aspects of pain and pain management, such as access to pain information, how they were coping and their medication use.
- Inclusive research practices
At the research stage, these subgroups should have been identified and considered during the production stage.
This creates a more thorough and inclusive process when it comes to analgesic development, which has the potential to contribute to a considerably higher clinical success rate.
Opening new revenue opportunities
From a business perspective, targeting rare or overlooked pain conditions can present a unique revenue stream for those developing new analgesics. It provides new opportunities that are not being considered by the wider industry.
Pharmaceutical companies developing drugs for rarer diseases can often receive considerable incentives such as tax credits, longer patient exclusivity and easier FDA review processes.
The status of rare drugs grants extends market exclusivity (by seven years in the US and 10 years in Europe), allowing businesses to sustain market dominance for longer periods.
Research⁴ shows that drugs for rare diseases can actually earn pharmaceutical companies nearly as much as drugs marketed to the public, despite treating far fewer patients.
For example, a drug for cystic fibrosis (which affects 30,000 Americans) made $5 billion over five years, compared to a drug treating Crohn’s disease and ulcerative colitis (affecting one million Americans), which made $5.5 billion.
This opens opportunities for proof of concept, as explored when we discuss diabetic neuropathy earlier in this article.
How Pain Cloud® can help
At Pain Cloud, we believe every pain patient should be treated based on the severity of their condition, not by how rare it is or how it is regarded by the industry.
Pain Cloud, a B2B platform created by EptivA Therapeutics and Infopoly, uses the first precision medicine approach to pain, creating more effective treatments, increasing clinical success and reducing costs.
The Pain Cloud approach to research, teamed with our proprietary Pain Landscape® database, allows us to match research targets to disease and significantly de-risk pain R&D, forming a positive chain reaction that results in faster, more efficient treatment for patients.
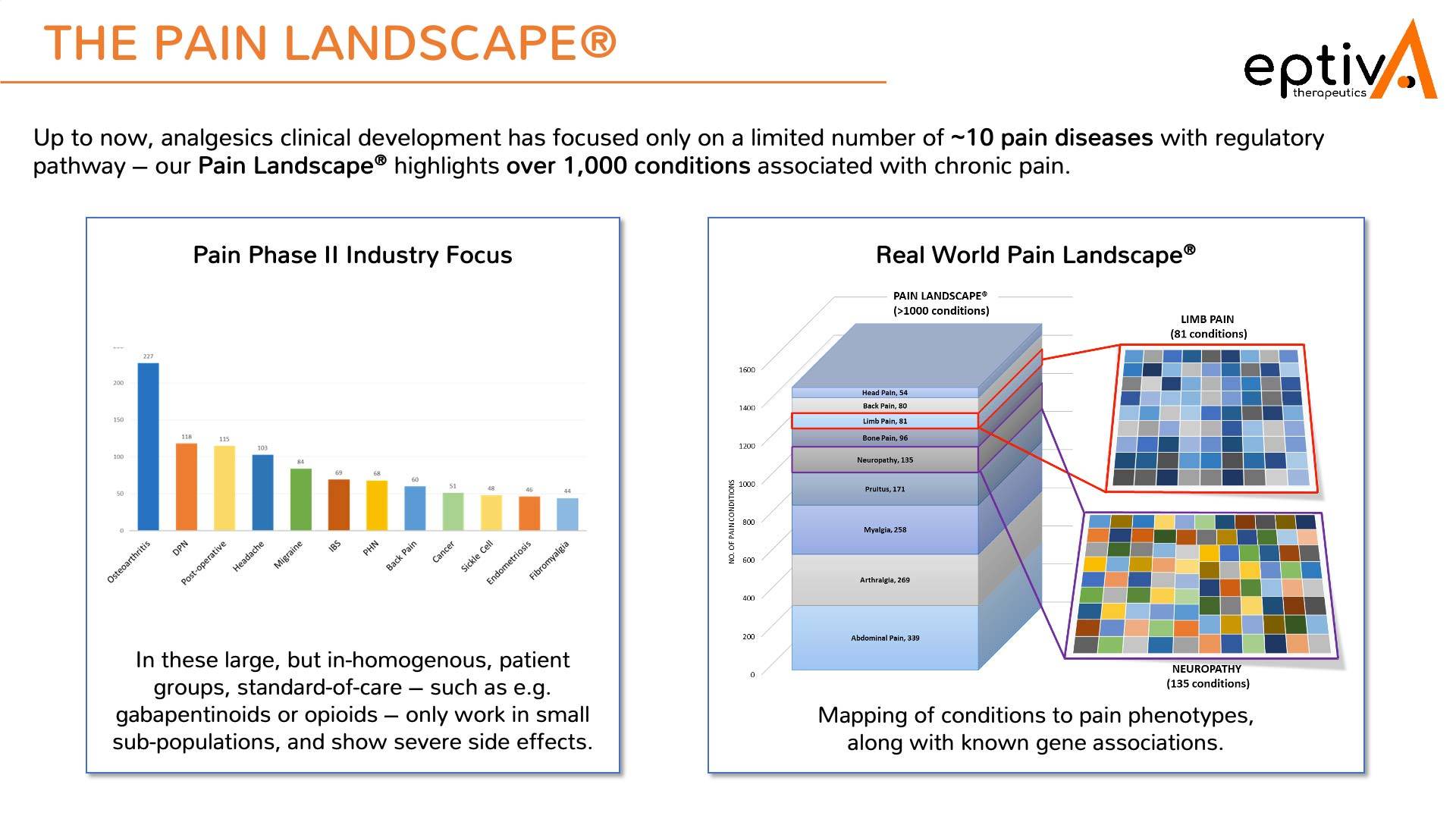
Our Personalized Analgesics® bioinformatic approach creates thousands of nodes of data via novel human-focused network biology, with the Pain Landscape highlighting over 1,000 conditions associated with chronic pain.
To find out more, read this article on how Pain Cloud changes the landscape of target validation and get in touch if you have any questions.
- Breivik, H., Collett, B., Ventafridda, V., Cohen, R., Gallacher, D. (2006). Survey of chronic pain in Europe: Prevalence, impact on daily life, and treatment. European Journal of Pain. 10(4), p.287. [Online]. Available at: https://onlinelibrary.wiley.com/doi/10.1016/j.ejpain.2005.06.009 [Accessed 15 August 2024].
- Odling-Smee, L. (2023). Chronic pain: the long road to discovery. [Online]. Nature. Available at: https://www.nature.com/immersive/d41586-023-00869-6/index.html [Accessed 15 August 2024].
- Widerström-Noga, E., Anderson, K.D., Perez S., Martinez-Arizala, A., Cambridge, J.M. (2018). Subgroup Perspectives on Chronic Pain and Its Management After Spinal Cord Injury. Journal of Pain. 19(12), pp.1480-1490. [Online]. Available at: https://www.sciencedirect.com/science/article/pii/S1526590018303547 [Accessed 15 August 2024].
- Tu S.S.Nagar S.Kesselheim A.S.Lu Z.Rome B.N. Five-Year Sales for Newly Marketed Prescription Drugs With and Without Initial Orphan Drug Act Designation. JAMA. 2023;329(18):1607–1608. doi:10.1001/jama.2023.3079