How Pain Cloud® Tackles the Challenges of Target Validation
Did you know that one in four people worldwide are suffering from chronic pain? Read on to find out how our B2B platform, Pain Cloud®, can help you improve your POS while putting patients first.
The chronic pain landscape
Chronic pain can vary in severity, be present all the time or be intermittent, and can happen anywhere in the body. It can greatly affect your work life, social life, and your ability to take care of yourself and others.
There are a wide variety of chronic pain conditions. Some of the most common include:
- arthritis and joint pain
- back pain
- neck pain
- pain linked to cancer
- headaches and migraines
- fibromyalgia and other muscle conditions
There are also hundreds of other less well-known conditions (including rare and orphan diseases) where patients are suffering from chronic pain symptoms.
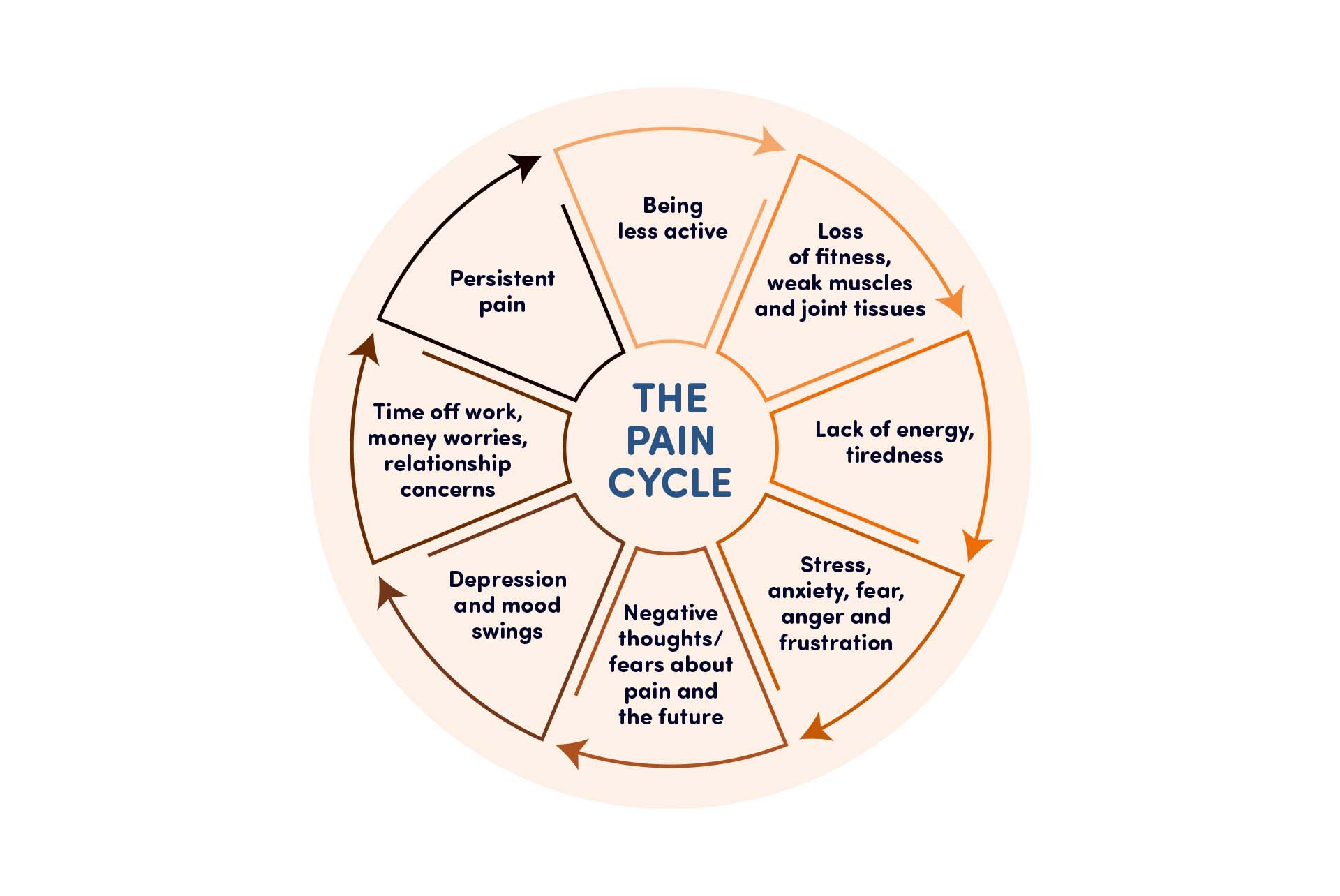
The lasting effects of chronic pain can lead to depression and anxiety and, in turn, insomnia, work absenteeism and a decrease in activity, leaving patients in a vicious cycle that’s difficult to break.
This makes the demand for effective and reliable analgesia all the more pressing. However, many pain patients are not receiving the medicine they need, and current treatments only work in small subpopulations with 99% of new pain research failing in clinical development.
Critical need for safe new analgesics
The current pain epidemic illustrates the urgent need for safe, new analgesics. This is becoming more crucial each year with an aging population prone to pain conditions.
The average R&D cost of one new drug is $2.5 billion for analgesics; the overall costs will be much higher as they fail in clinical development more often than other medicines. Inadequacies in R&D and target validation are costing health organizations billions of dollars. Even with suboptimal treatments, the pain market will reach $91 billion by 2027.
There is a desperate need for new, more effective pain treatments – so why are we struggling to develop new analgesics?
Problems faced in developing new analgesics
- the pain R&D model has not evolved in over 20 years; it relies on non-translatable preclinical models
- clinical development tends to focus on a limited number of pain diseases with regulatory pathways, rather than identifying the optimal disease for each target
- translational research is costly and time-consuming, with limited current options
- difficulties emerge as relevant human tissue for translational studies is often unavailable
- 99% of new pain research currently fails in clinical development
Here are some examples of first-line treatments for neuropathic pain that have proven to be imprecise:

This data demonstrates that standard treatments only work for a minority of patients and that first-line treatments for neuropathic pain are imprecise, with only one in ten patients receiving any benefit.
We can change this
EptivA’s innovative B2B platform, Pain Cloud®, can radically improve how pain research takes place by linking targets to the optimal pain group more precisely.
Unpacking the basics of target validation
One of the major reasons underpinning the current issues in the pain landscape is a lack of target validation for existing and new analgesic mechanisms.
- Target validation in pharmaceutical research and development (R&D) is a critical step in the discovery of a new drug.
- Simply put, it’s the crucial first step in the discovery of a new drug, involving several techniques performed to demonstrate that a drug is engaging the target to produce the desired therapeutic effect.
- This is usually a protein or gene that interacts with, and whose activity is modulated by a particular compound.
- The target needs to be relevant to the human disease phenotype and should be amenable to therapeutic modulation.
- It is essential to de-risk a drug discovery program.
Efficient target validation is difficult as systematic use of human tissue has not been implemented and the current approaches to translational research are extremely expensive and labor intensive. If translational work is concluded within its limitations, the subsequent focus often is on the target instead of the modulation of pathways which drive the efficacy.
The first precision medicine approach to pain treatment
At EptivA, we understand that a radical approach is needed to improve the target validation landscape.
That’s why we have created a radical new R&D platform to transform and de-risk pain drug development. Our unique precision medicine approach will create more effective, non-abuse-potential treatments and increase clinical success.
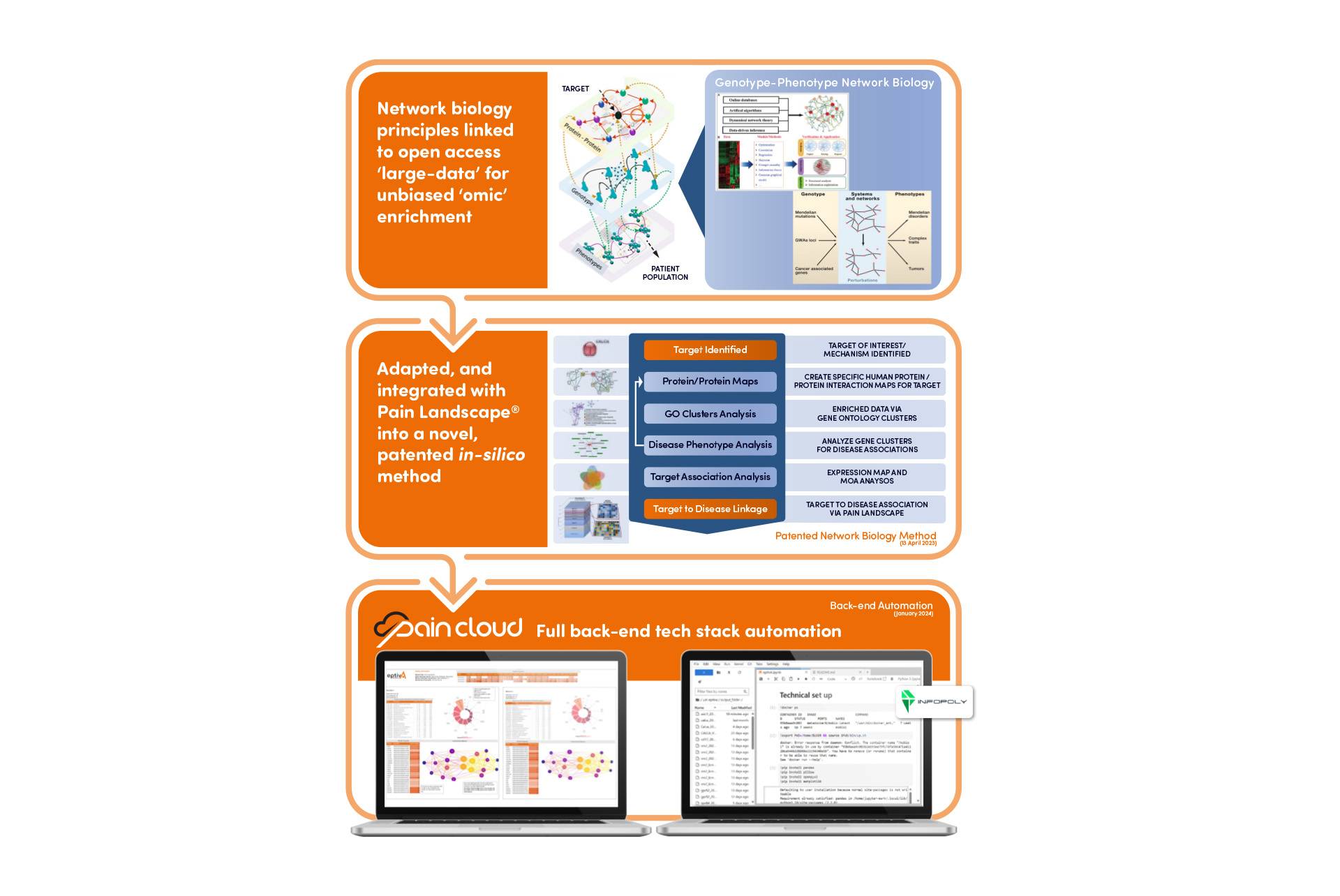
To mitigate the challenges posed by traditional pain research and development (R&D), eptivA Therapeutics, in partnership with Infopoly, has launched a B2B platform, Pain Cloud®, using the Personalized Analgesics® in-silico network biology research to help scientists validate their research target and identify optimal patient groups for clinical development.
Pain Cloud® is the first precision medicine approach to pain with the aim of creating more effective treatments and increasing clinical success.
We have created a novel in-silico Personalized Analgesics® research method for linking protein research targets to over 1000 pain-related conditions from our Pain Landscape® . The methodology uses a unique algorithm of high-quality molecular biology databases which focus on human-relevant data allowing Pain Cloud® to match targets more precisely to optimal pain patient groups.
This diagram highlights how Personalized Analgesics® in-silico network biology research compares to traditional methodologies.
Our partnership with Infopoly
By partnering with digital technology experts Infopoly, Pain Cloud® has become an automated platform with the development of a back-end tech stack via integrated novel software.
The network biology algorithm can now be operated, automatically accessing huge data sets from high-quality molecular databases, providing unbiased analysis linking research targets to optimized disease phenotypes.
Our platform has already been validated with a wide range of mechanisms, from established medicines to novel research targets, through our research collaboration with Bayer Pharmaceuticals.
We have analyzed a range of targets including:
- CGRP (clinically established targets)
- TRPV3 (pre-clinically established targets)
- GPR18 (orphan GPCR)
- SV2a (pre-clinical false positive)
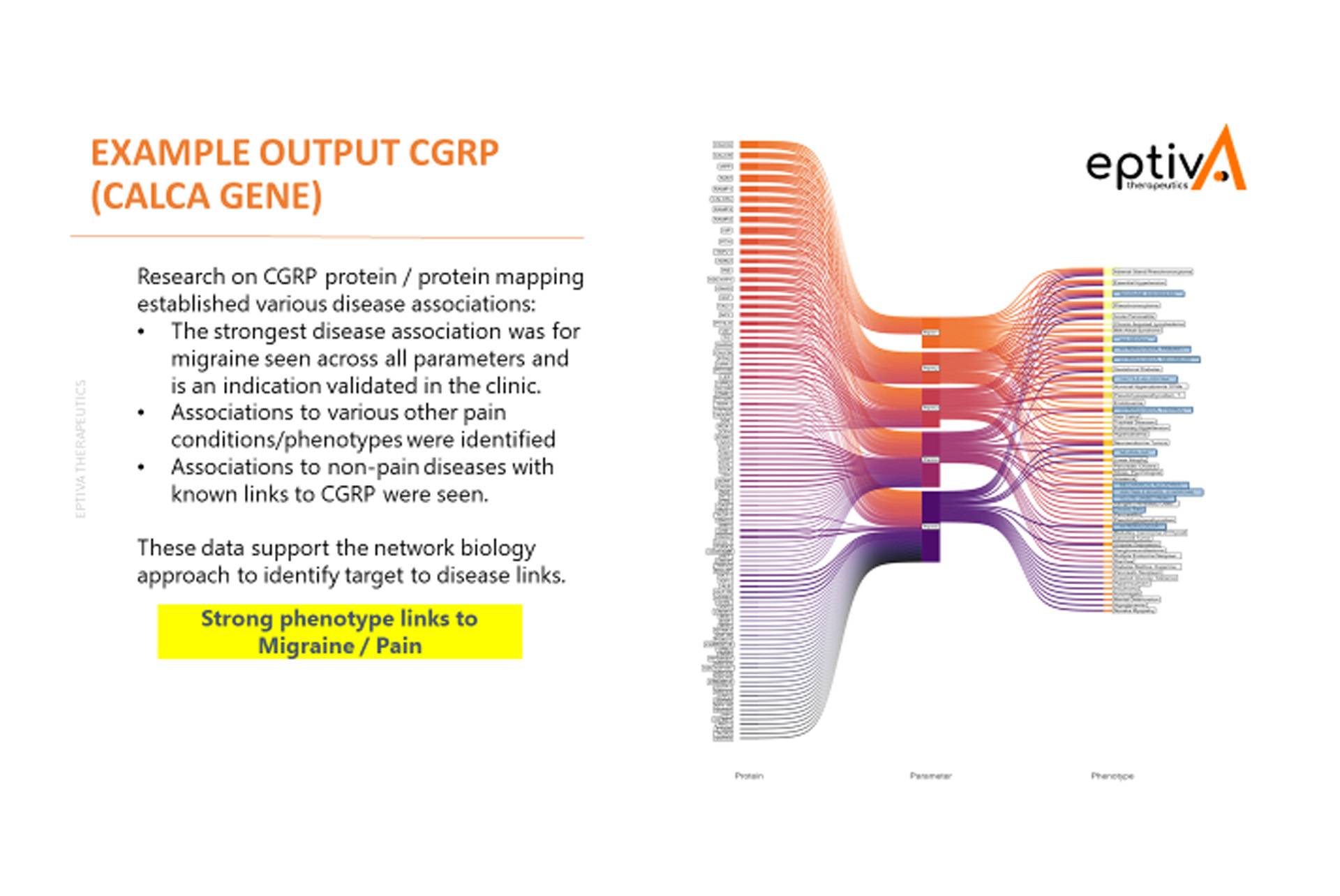
Here is an example of the summary output from the new Pain Cloud® software. It highlights how multiple proteins associated with CGRP, an established medicine for migraine, were used for the network biology analysis that identified core disease phenotypic links for this research target. The strongest links identified for the CGRP protein included migraine and allodynia, which are validated by its clinical use.
“Infopoly have further developed our novel methodology, enabling an Advanced Pain Management Solutions B2B platform. By utilising Pain Cloud®, researchers can avoid the challenges of cost-intensive traditional R&D methodologies with a streamlined process, precisely matching targets and mechanisms to specific patient groups and saving time.”
