The Benefits of Drug Repurposing and How Pain Cloud® Can Help
Despite chronic pain being so prevalent across the Western world, treatment and management are still suboptimal, with the industry failing to innovate novel treatments as demonstrated by a 99% clinical development failure rate. However, repurposing is a viable avenue to validate existing medicines as new pain targets and push through to successful new treatments.
In this article, we explain how repurposing can lead to faster development times, increased patient safety and a higher chance of success in analgesic development.
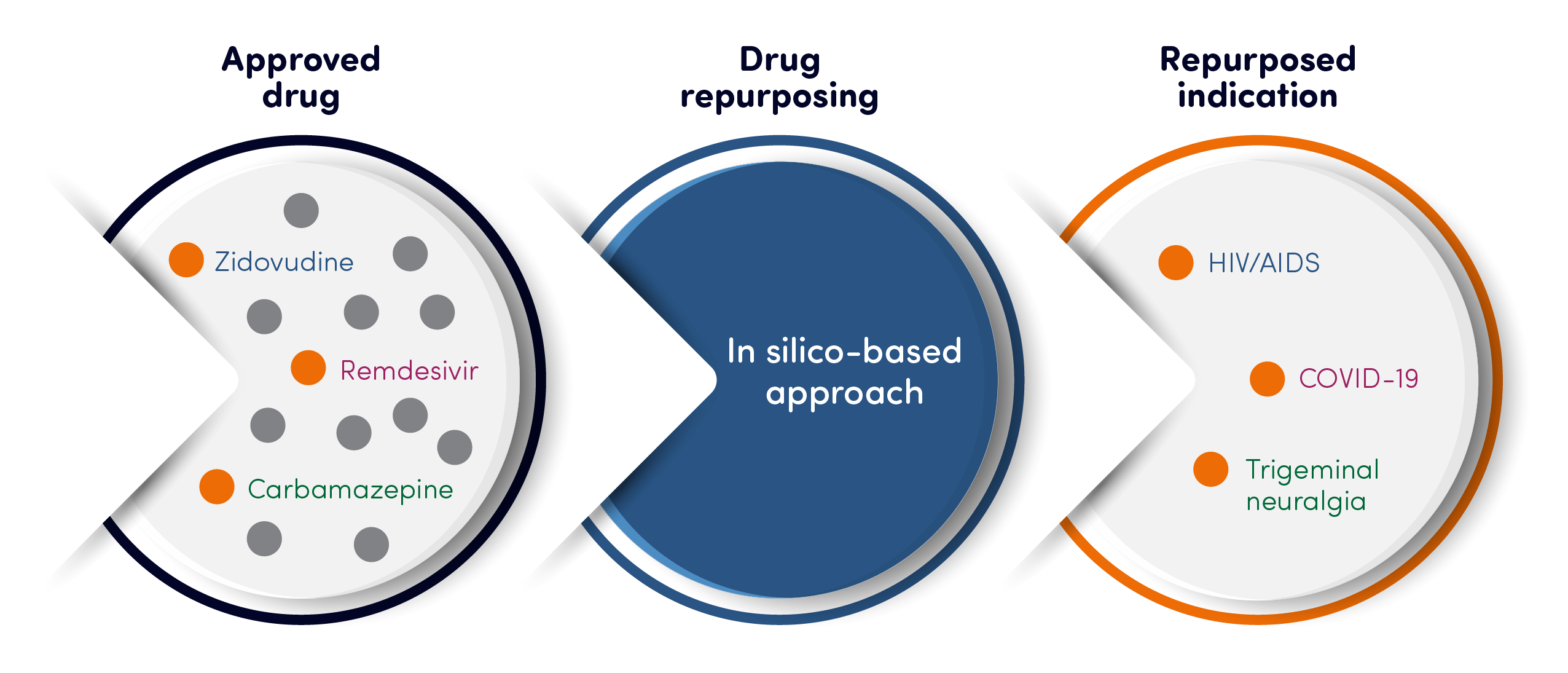
Drug repurposing: explained
Typically, medicines are developed to target specific proteins that are known to be involved in disease. Thousands of these proteins, which cover a wide range of therapeutic areas and modalities, have been developed into research targets, with some of them progressing to become medicines.
Most of the medicines target specific patient groups with areas such as oncology, immunology and metabolic diseases. As the industry learns more and more about different disease areas, we can discover potential links between medicines and their efficacy for other conditions.
For example, in pain medicine, we now understand that the underlying biology and pathology are more complex than just misfiring nerves, inflammation and trauma.In many cases, the immune system is greatly involved, and specific immune cells may initiate and maintain certain pain conditions. In this instance, it is possible that existing immune modulator medicines have a positive impact on certain pain conditions.
Our increased knowledge of specific cells can also provide further insights and create new opportunities. For example, keratinocytes (skin cells which were thought to only protect the skin) actually contain important pain mechanisms and initiate a pain response themselves. This discovery allows us to explore the possibility of identifying dermatological treatments as potential analgesics.
There are also numerous examples of interesting protein research targets that made it into clinical development and have been shown to be safe – however, they failed due to a lack of efficacy.
In many of these cases, they may not have been explored within the most optimal patient groups and could bring about new options for new therapies if the right match had been identified.
Part of the failure could be the use of a limited patient groups. However, Pain Cloud® is based upon EptivA Therapeutics’ database of over 1,000 pain-related conditions.
This allows businesses developing new analgesics to identify disease associations to novel pain conditions with improved accuracy.
The potential benefits
- Faster development times
Drug repurposing can see success more quickly than the regular development of new analgesics because targets are already in market or clinical development.
This means that potential treatments can become available to patients more rapidly.
- Patient safety
Unlike novel treatments, repurposed drugs have existing safety data, potentially reducing safety risks for patients.
- Cheaper development
As the clinical safety data already exists and streamlined clinical development plans can be used, development costs can be significantly cheaper.
- Increased success rate
Drug repurposing can improve the success rate of new analgesics, which currently sits at just 1%.
It can bypass many of the challenges of traditional drug development and patients are often more willing to take part in clinical trials.
This study1 researched how many approved drugs could target peripheral sensory neurons and neuroinflammation of the spinal cord – and several of these approved drugs showed promising antihyperalgesic or analgesic effects when it came to reducing stress of peripheral sensory neurons or targeting inflammation of the spinal cord.
There are also existing examples of drugs developed in another areas that eventually demonstrated efficacy in chronic pain. For example, gabapentin was originally developed and marketed as an anti-epileptic. However, research demonstrated its potential in neuropathic pain conditions which was confirmed in extensive clinical trials and pain became its main therapeutic use.
There are some challenges associated with drug repurposing, such as typically having difficulty developing patent protection. This can put off companies who can find difficult building ROI in such cases.
However, new technology analysing novel associations between targets and diseases may provide data to support novel IP protection to support development. In this way we believe repurposing can really provide businesses with the chance to improve their success rate and help pain patients access the treatment they need in a faster, safer and more efficient way.
In 2022, we used the Pain Cloud® platform to identify a novel link between cholecalciferol and specific pain conditions. These data were used to support a published method of use patent, which forms part of our IP and protection strategy for this new treatment approach.
We hope that this will provide a new choice of analgesic to patient groups with limited or no treatment options.
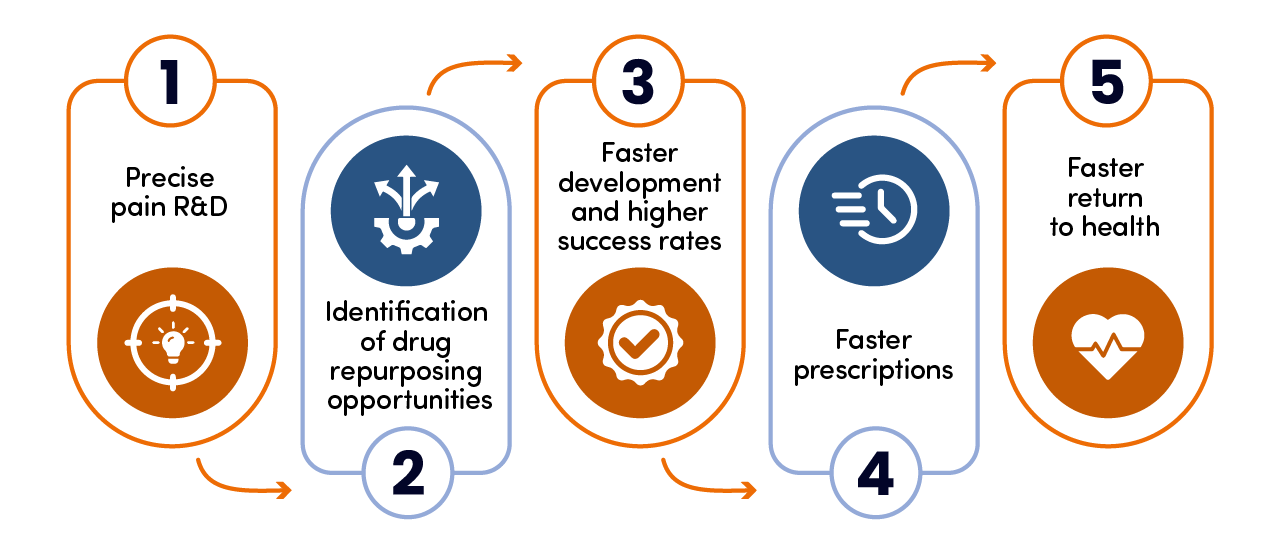
How Pain Cloud® can help
Any successful pain drug repurposing needs to be centered around accurate research based on a large, versatile database.
Traditional research is often based on built upon a relatively small number of pain conditions. However, there are hundreds of conditions associated with pain which could provide the right patient groups for analgesic targets.
Our Personalized Analgesics® bioinformatic approach creates thousands of nodes of data via novel human-focused network biology, linked to our proprietary Pain Landscape®.
This large database gives businesses developing new analgesics the best possible chance of finding new avenues for repurposing and providing a better future for pain patients.
Read more about our solutions and feel free to get in touch if you have any questions or points to discuss.
- Sisignano M, Gribbon P, Geisslinger G. Drug Repurposing to Target Neuroinflammation and Sensory Neuron-Dependent Pain. Drugs. 2022 Mar;82(4):357-373. doi: 10.1007/s40265-022-01689-0. Epub 2022 Mar 7. PMID: 35254645; PMCID: PMC8899787.
- Dahlhamer J, Lucas J, Zelaya C, Nahin R, Mackey S, DeBar L, et al. Prevalence of chronic pain and high-impact chronic pain among adults—United States, 2016. MMWR Morb Mortal Wkly Rep. 2018;67(36):1001–6.
- -. (2017). Chronic pain in adults 2017. [Online]. Public Health England. Available at: https://assets.publishing.service.gov.uk/media/5fc8c6b78fa8f547585ed7f3/Chronic_Pain_Report.pdf [Accessed 5 September 2024].
